Research Interests:
- Biological fluid dynamics
- Cerebrospinal fluid, including the glymphatic system
- Lymphatic system
- Blood flow in the liver
- Traumatic brain injury
- Mechanisms of injury related to fluid mechanics
- Disruption to glymphatic/lymphatic function
- Soft porous media
- Deviations from Darcy's law
- Fluid-structure interaction
- Dynamical systems approaches to turbulence
- Exact coherent structures
- Lagrangian coherent structures
We utilize the following tools/approaches:
- Particle tracking and particle image velocimetry to quantify fluid flow
- Computational fluid dynamics
- In-house finite difference codes
- Nek5000 (spectral element code)
- Lattice Boltzmann Method
- In vivo experiments in mice
- Microfluidics and in vitro models
- Machine learning and data-driven modeling
Fluid Mechanics of the Glymphatic System
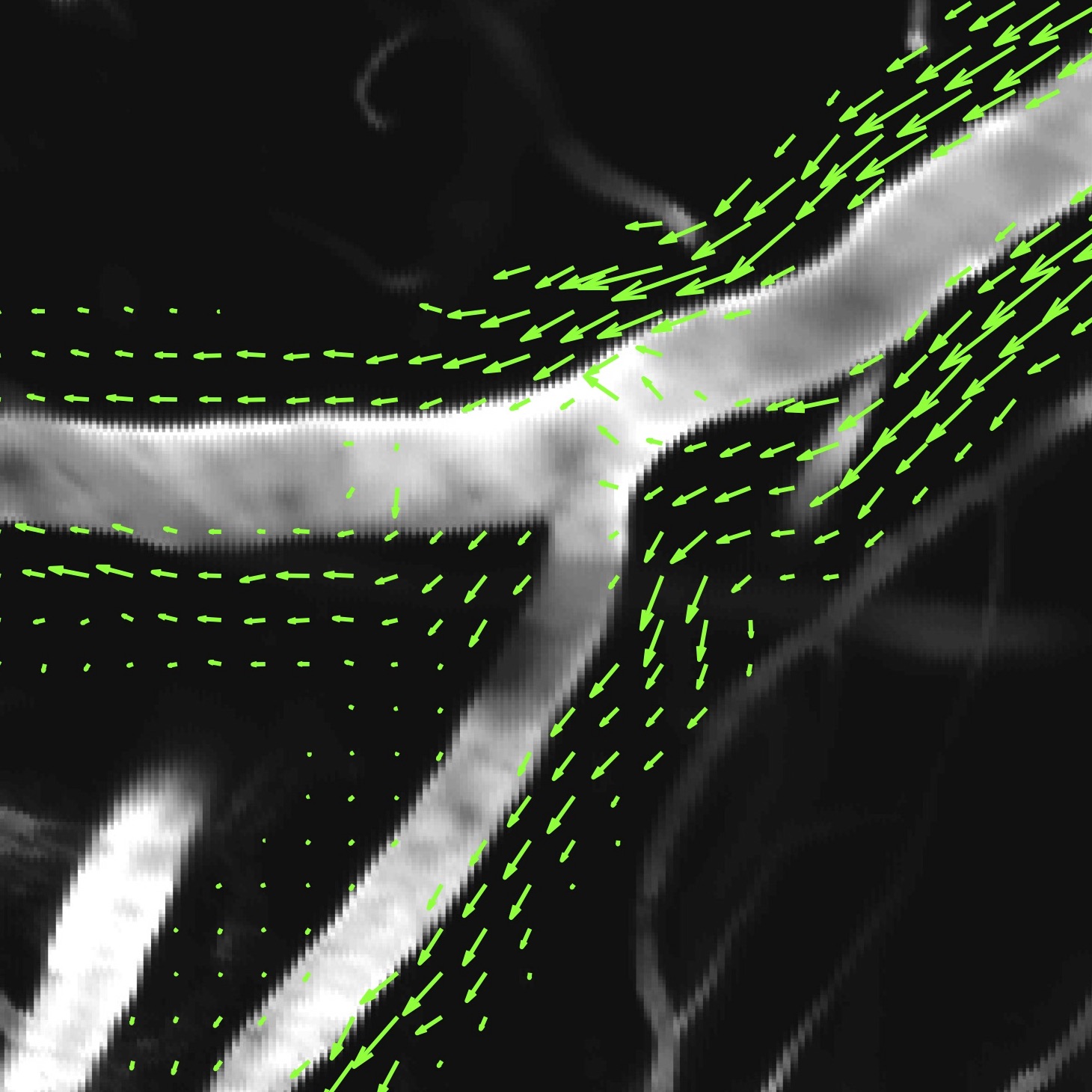
Within the last decade, there has been a greatly increased appreciation for the vital role that cerebrospinal fluid (CSF) plays in maintaining brain health. This is especially inspired by recent breakthroughs in experimental measurement techniques which have enabled in vivo measurement of CSF flowing into the brain (see figure at right). A growing body of research demonstrates that CSF flow through the brain provides an important mechanism for metabolic waste removal and that disruption to this system is directly involved in the cognitive decline associated with many neurological disorders, including Alzheimer’s disease, stroke, traumatic brain injury, and more.
This clearance pathway in the brain, dubbed the glymphatic system in 2012, includes CSF flow through perivascular spaces (PVSs)—annular channels around blood vessels—in the brain. Interestingly, this flow is largely inhibited during wakefulness and only turns on during sleep, perhaps providing a reason for the necessity and restorative effect of sleep. There are currently a tremendous number of open questions that engineers are uniquely well-suited to answer, many of which the Tithof lab and collaborators are actively pursuing:
- What drives the flow? Many experimental studies suggest the flow is driven by arterial pulsations via peristalsis, but multiple prior, highly idealized simulations have cast doubt on this hypothesis. Relevant publications: Mestre, Tithof, et al 2018
- What is the precise pathway CSF flow follows? Technical limitations currently prevent high-resolution, in vivo measurements throughout the entire brain. Numerical modeling provides a promising approach for predicting brain-wide transport, bridging observations in different experiments, and developing experimentally-testable hypotheses. Relevant publications: Tithof et al 2019, Troyetsky et al 2021, Tithof et al 2022
- How does this system go awry during acute neurological conditions (e.g., stroke, traumatic brain injury)? In particular, many such conditions initiate waves of spreading depolarization (irregular heightened then inhibited neuronal activity) which have recently been demonstrated to disrupt the glymphatic system. Relevant publications: Mestre et al 2020, Du et al 2021, Hussain et al 2022 (under review)
- Can this pathway be utilized as a novel route for drug delivery? The brain is unique from the rest of the body in that it strictly regulates what is able to pass back and forth between the blood and brain tissue (this is called the blood brain barrier). It may be possible to deliver drugs (e.g., cancer treatments) to the brain more effectively via CSF flow. Relevant publications: Plog et al 2018
The Tithof lab is interested in:
- Numerical simulations and modeling. Mahsa is currently developing simulations of CSF flow through perivascular spaces to investigate the driving mechanisms. Saikat is developing simulations of spreading depolarization to gain insight into the competing sources of CSF flow disruption.
- In vivo experiments. Danny is currently measuring and modeling flow through lymphatic vessels (a route through which CSF exits the skull), in collaboration with Professor Hussain at University of Rochester Medical Center. At UMN, colleagues in Professor Kodandaramaiah's research group have recently developed techniques for implanting transparent polymer skulls in mice, enabling prolonged imaging of most of the dorsal cerebral cortex. Cooper is pursuing mouse experiments that leverage such technologies.
- In vitro models. Many of the open fundamental questions related to mechanisms driving CSF flow can be investigated using non-living in vitro models of the glymphatic system. Jake has developed and is probing such an experiment.
Liver Modeling to Improve Surgical Outcomes
The liver is the only organ with the ability to regenerate, enabling full recovery after tumor resection or living donor liver transplantation. However, the liver has extraordinarily complex branching vasculature, and consequently, clinicians are faced with challenging surgical decisions that may lead to complications or even liver failure, which has a high rate of mortality. We are developing a versatile and computationally efficient lumped parameter numerical model that can simulate blood flow through the entire human liver in a matter of minutes on a standard laptop. This model is being developed in collaboration with Dr. Joseph Sushil Rao and Professor Timothy Pruett (Department of Surgery, University of Minnesota), with the long-term goal of introducing a versatile computational tool that will assist clinicians in operative planning to improve surgical outcomes.